Luis Alvarez envisions a day when therapeutic proteins that hasten cellular development can be delivered with high precision and long duration to drive the regeneration of entire tissues, and when war-injured soldiers don’t have to undergo delayed amputations because their broken limbs won’t heal due to incomplete tissue regeneration.
These are realistic dreams for Alvarez, a 20-year Army veteran and retired lieutenant colonel whose fellow warriors’ experiences dealing with combat injuries motivated him to pursue a doctorate in biological engineering at the Massachusetts Institute of Technology. In 2016 he spun out Theradaptive, a regenerative medicine company, based on his work at MIT.
During his doctoral research, Alvarez created a protein-engineering platform that enables proteins to bind to materials found in surgical implants with very high affinity. This results in a bioactive coating similar to a paint that directs and enhances cellular behavior. Alvarez’s technology keeps therapeutic proteins at the implant site working where they belong, thereby avoiding off-target effects elsewhere in the body which can lead to unwanted and detrimental side effects. The applications of this technology range from regenerative implants that can regrow tissue to persistent, localized delivery of cancer-killing therapies.
“The core of our technology is based on the idea that you can take proteins that already occur in the body and re-engineer them so that they can be loaded onto an implant without the use of chemical ligation,” said Alvarez, CEO of Theradaptive. “In order to do that, we’ve created a method to change the sequence of the amino acids in the protein without changing the function of the protein.”
Alvarez and his team can also tune the proteins to control their release rate.
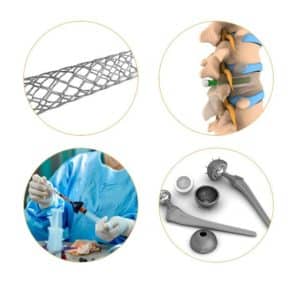
“We have applied our modification to over a dozen proteins with therapeutic applications in regenerative medicine, arthritis and oncology, and have demonstrated the ability to coat various implant types, including meshes, spinal implants, stents, electrospun scaffolds and porous wafers,” Alvarez told Xconomy magazine in 2020. “For example, in the area of mesh repair, we have two proteins that enhance tissue repair locally so that the mesh can be resorbed and eventually replaced by native tissue. In the area of targeted chemotherapy, we are able to safely bind very high doses of therapeutic proteins and release them locally at a known rate while avoiding off-target effects.”
By administering the therapeutic agent locally, Alvarez believes it may become possible to do away with systemic side effects like nausea, neuropathy and cytokine storms associated with chemotherapy and immunotherapies or unwanted bone growth associated with cervical spinal fusion.
As a small company, the team at Theradaptive had to pick one initial therapy to focus on. They chose an orthopedic procedure, spinal fusion, as the best initial business case that would also result in treatments for bone repair of the extremities and craniomaxillofacial repair while using the same product design. This efficient use of the same protein and implant enables the company to quickly expand into additional clinical indications.
Theradaptive also has active programs in immuno-oncology, cartilage and dermal repair. To date, the company has raised over $30 million with $15 million from the U.S. Department of Defense and from the Maryland Stem Cell Research Fund to develop products based on its platform.
Alvarez’s novel approach—using biologically active surgical implants to restore or treat tissue—may help usher in a new era in regenerative medicine.
“For the first time we’ll begin to see the precisely targeted use of proteins to potentiate cells to actually regenerate tissue and not have to rely on inert implants,” said Alvarez. “In the next 12 months, we’ll begin to see the fruits of this work and the result will be actual regenerative medical treatments available to patients who have no other options.”
This aspiration came one step closer to fruition in 2021, when the U.S. Food & Drug Administration granted Breakthrough Medical Device designation to Theradaptive’s OsteoAdapt SP Spinal Fusion implant for transforaminal lumbar spinal fusion to treat degenerative disc disease, spondylolisthesis and retrolisthesis. The FDA subsequently granted the company two more breakthrough designations for posterolateral and interbody spinal fusion this year, meaning they currently hold three breakthrough designations in the spine, more than any other company in the spinal fusion space.
The OsteoAdapt breakthrough designation means the FDA will give Theradaptive priority review and two-way communication about device development and clinical trial protocols. OsteoAdapt is scheduled to begin Phase I/II clinical trials in 2023 and will begin enrolling in both the U.S. and Australia.
Alvarez and his team have also tapped into regulatory support via the company’s membership in BioFabUSA, a program of the Advanced Regenerative Medicine Institute (ARMI). They credit Richard McFarland, ARMI’s chief regulatory officer who joined ARMI after 17 years at the FDA, for his guidance.
“One of the most tangible benefits of membership has been the type of support that you normally couldn’t find elsewhere,” Alvarez said.
As well as providing regulatory guidance, BioFabUSA can also help members automate their processes. It has an embedded executive from automation company Siemens and custom engineering and integration support from DEKA Integration Services Corp. (DISC). DISC is a unit of DEKA Research & Development Corp., which was founded by the same person who leads ARMI: Dean Kamen.
Alvarez sees potential for automation in the manufacturing process for OsteoAdapt, which is made by adding Theradaptive’s bone morphogenetic protein variant, AMP-2, to an electrospun cotton-like implant, ReBOSSIS, from Japanese company ORTHOReBIRTH.
“We have a GMP manufacturing step where you bind the regenerative protein to the implant and package the final product: that whole process could go into automation,” he said. “As we mature that process it would be interesting to explore some of the automation that BioFab could offer.”
With the right support in place, Alvarez and his team are well placed to harness all the life-changing potential of the Theradaptive platform and begin to scale, bringing new, regenerative therapies to patients who have no other treatment options.