Over the last several years, there have been considerable advancements in biotechnology that bring the promise of a radically different way of providing healthcare in the future. Rather than treating the symptoms of disease alone, the field of regenerative medicine seeks to resolve the underlying ailment with gene and cell therapies, or tissue-engineered products intended to augment, repair, replace or regenerate organs, tissues, cells, genes, and metabolic processes in the body.
When Lynne Frick joined Cell X Technologies as CEO in 2021, the organization had already partnered with BioFabUSA, a program of the Advanced Regenerative Manufacturing Institute (ARMI) and was exploring ways to leverage their expertise to support the regenerative manufacturing process.
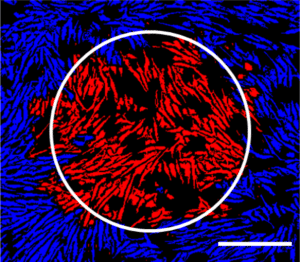
“I attended a Meeting in the Millyard event in the fall of 2021 almost immediately after coming on board with Cell X and it was there that I heard Doris Taylor give a talk on her ‘ghost heart’ project, striving to provide personalized human hearts by augmenting pig heart scaffolds with human cardiomyocytes. My immediate thought was that Cell X could have a role in helping her define the critical quality attributes of the cardiomyocytes, so we got to talking. We then applied for and received a mini grant through ARMI after defining a project that would help us both. Since then, we have been working together to find a way to industrialize the manufacture of cardiomyocytes so that the process is more robust and predictable.”
The Cell X platform helps scientists and cell manufacturers enable regenerative medicines by using responsive robotic automation to improve process reproducibility while addressing challenges in defining product quality through artificial intelligence. This stands in contrast to the manual workflows and subjective decision making of the status quo, which are not sufficiently reproducible or scalable. Powered by the Cell X Device and Cell X Colonyze™ software, the platform helps technicians by improving decision-making, standardizing inconsistent methodologies, and automating manual processes. In speaking with Frick about how this technology can support the field of regenerative medicine, she is confident that it will help enable further breakthroughs in the field.
“A lot of products have failed not because of their efficacy, but because the product was not characterized up to the standard of what the FDA required. The way much of the industry is producing these cells now involves a highly skilled scientist in a hood with a pipette, looking through a microscope selecting and advancing particular cells based on visual decision making, which although may be perfectly good day to day with one person, it is not reproducible across different people or different facilities. The Cell X platform allows us to monitor and act on the biology in the dish throughout the entire process. We have a way to document everything, with a timestamp, from the very first founding progenitor cell all the way through the entire process. Our industry requires 21 CFR part 11 compliance, and we have designed our software to meet this standard. We are also able to follow cell colonies over time, biopsy them as we go and then annotate these images with outside information from other assays, linking all that information. We then apply machine learning artificial intelligence so that we’re building a process-specific algorithm resulting in fully automated the cell differentiation and expansion process. This helps to solve the challenge of process reproducibility and robustness, resulting in a predictable product.”
While the Cell X platform aims to better standardize and stabilize processes, the fields of regenerative medicine and manufacturing are still faced with the same basic challenges as any form of production when it comes to defining industry-wide standards. While there are currently ways of taking measurements, they are not necessarily indicative of the phenotype of the cell being produced. To overcome this challenge, the industry must find ways to make assays faster and more efficient, and also more indicative of what is happening with the specific phenotype of cell being produced. Frick is optimistic about potential breakthroughs in the not-so-distant future.
“Having iPS-derived therapeutics succeed in the clinic is a big goal. We are collaborating with a customer that is addressing childhood blindness. To have a child lose their sight at age four or five, then be able to take a piece of their skin, reprogram the cells, create neuronal cells on the Cell X Device, and then create the retinal organoids after CRISPR-correcting the gene causing the problem is pretty exciting. Another interesting application is Parkinson’s Disease: instead of trying to treat the symptoms, you’re actually aiming at a cure by producing neurons that can be engrafted and produce dopamine. That’s what I see in the near future: engineering things to change the course of the disease as opposed to merely treating the symptoms.”